NorthEast Monitoring LX Sleep Software
Questions? Speak with a Product Specialist!
Let LX® Sleep be the answer to your dreams with in-home, overnight, unattended, multi-parameter, sleep study for detection of OSA (Obstructive Sleep Apnea). This convenient HST (Home Sleep Test) device provides a conforming methodology for multi-parameter, overnight, unattended sleep study that is described in CMA’s NCD (National Coverage Decision) CAG-00093R2. LX® Sleep software applies proprietary algorithms to data collected by NorthEast’s OxyHolter to provide a simple alternative to the PSG sleep lab experience to accurately identify OSA (Obstructive Sleep Apnea).
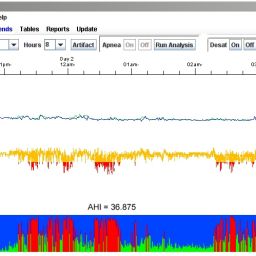
LX® Sleep defines epochs of normal versus SDB (Sleep Disordered Breathing). Then it performs pattern recognition on ECG and SpO2 signals; and applies a linear discriminant analysis and an age-based classifier to derive an AHI (Apnea Hypopnea Index). An AHI < 5 represents no clinically significant apnea; 5 < AHI < 15 represents mild apnea; AHI >15 represents moderate-to-severe apnea.*
*As per Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2); March 13, 2008.
| Holter Brand | |
|---|---|
| Holter Accessory Type |